Abstract
Introduction: DARA is approved across lines of therapy for multiple myeloma, including in combination with standard-of-care regimens for NDMM. The CXCR4 receptor antagonist plerixafor is used in conjunction with granulocyte colony-stimulating factor (G-CSF) to increase stem cell mobilization for autologous stem cell transplant (ASCT) and can be given by upfront decision or as a rescue strategy. The phase 2 randomized GRIFFIN study (NCT02874742) evaluates frontline DARA in combination with lenalidomide, bortezomib, and dexamethasone (D-RVd) in transplant-eligible NDMM. In the primary analysis, more pts undergoing stem cell mobilization/collection in the D-RVd group received plerixafor compared with the RVd group (69.5% [66/95] vs 56.3% [45/80]) (Voorhees PM, et al. Blood. 2020). The phase 2 MASTER study (NCT03224507) evaluates DARA plus carfilzomib, lenalidomide, and dexamethasone (D-KRd) in transplant-eligible NDMM (Costa LJ, et al. EHA Library. 2020). Here, we present a summary of stem cell mobilization, collection yields, and ASCT data following frontline DARA-based induction therapy in GRIFFIN and MASTER.
Methods: Eligible pts had NDMM and were candidates for ASCT. In GRIFFIN, pts were randomized 1:1 to receive D-RVd or RVd. Pts received 4 induction cycles (21 days) of lenalidomide (R; 25 mg PO on Days 1-14), bortezomib (1.3 mg/m 2 SC on Days 1, 4, 8, and 11), and dexamethasone (d; 40 mg PO QW) ± DARA (16 mg/kg IV QW in Cycles 1-4). After Cycle 4, pts underwent stem cell mobilization with G-CSF ± plerixafor, per institutional standards; if unsuccessful, chemo mobilization was permitted. Pts then received ASCT and subsequently 2 consolidation cycles (21 days) of D-RVd or RVd followed by maintenance therapy with R ± DARA. In the single-arm MASTER study, pts received 4 D-KRd induction cycles, ASCT, and 0, 4 or 8 D-KRd consolidation cycles followed by maintenance therapy with R, based upon achievement of minimal residual disease-negativity. In each 28-day cycle, all pts received carfilzomib (20/56 mg/m 2 IV QW), R (25 mg PO on Days 1-21), d (40 mg PO or IV QW), and DARA (16 mg/kg IV QW for Cycles 1-2, Q2W for Cycles 3-6, and Q4W for Cycles 7+). Mobilization was with G-CSF ± plerixafor as per institutional standards.
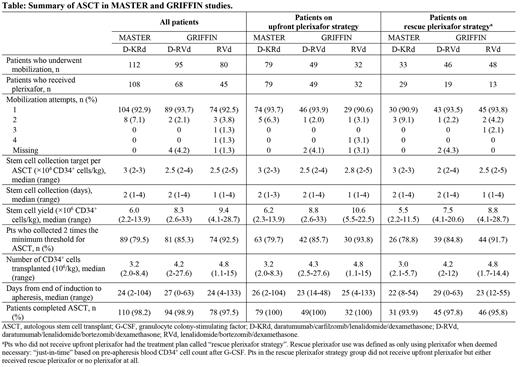
Results: In GRIFFIN, among 207 (D-RVd, n=104; RVd, n=103) randomized pts, 91.3% (n=95) of D-RVd pts and 77.7% (n=80) of RVd pts underwent stem cell mobilization; of those mobilized, 98.9% (n=94) and 97.5% (n=78) underwent ASCT, respectively. In MASTER, 123 D-KRd pts enrolled and at last follow-up, 91.1% (n=112) underwent stem cell mobilization; of those mobilized, 98.2% (n=110) completed ASCT. In GRIFFIN, 46.3% (n=81) of mobilized pts received plerixafor upfront (D-RVd, 51.6%, n=49; RVd, 40.0%, n=32), and 18.3% (n=32 pts) received rescue plerixafor (D-RVd, 20.0%, n=19; RVd, 16.3%, n=13). In MASTER, 70.5% (n=79) D-KRd pts received upfront plerixafor and 25.9% (n=29) received rescue plerixafor. Median CD34 + cell yield was 8.3 × 10 6/kg for D-RVd and 9.4 ×10 6/kg for RVd in GRIFFIN, 6.0 ×10 6/kg for D-KRd in MASTER, and was numerically higher for pts who received upfront plerixafor. Median days for stem cell collection was 1 for pts receiving RVd and 2 for those receiving D-RVd or D-KRd. Median transplanted CD34 + cell count was 4.2 ×10 6/kg for D-RVd and 4.8 ×10 6/kg for RVd in GRIFFIN, and 3.2 ×10 6/kg for D-KRd in MASTER. In GRIFFIN, 93.7% of D-RVd pts and 98.8% of RVd pts reached the minimum institutional CD34 + threshold to perform a single ASCT, which was comparable to results in MASTER (95.5% of D-KRd pts) after first mobilization attempt; 85.3% of D-RVd pts, 92.5% of RVd pts, and 79.5% of D-KRd pts collected 2 times the minimum threshold of stem cells. Additional data by upfront and rescue plerixafor strategies are shown in the Table.
Conclusion: The addition of DARA to proteasome inhibitor/immunomodulatory drug/dexamethasone-based induction therapy has a modest impact on stem cell mobilization, with a lower yield of stem cells and higher median number of days required for collection. Nonetheless, pts were able to undergo transplantation, and most pts collected sufficient stem cells for 2 transplants. Pts who received plerixafor by an upfront decision had numerically higher stem cell yields than pts who received plerixafor by a rescue strategy. An upfront plerixafor strategy for pts receiving DARA-based quadruplet induction therapy should be considered with allowance for additional days of apheresis as needed.
Chhabra: GSK: Honoraria. Costa: Janssen: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Speakers Bureau; Karyopharm: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau. Kaufman: BMS: Consultancy, Research Funding; Fortis Therapeutics: Research Funding; Roche/Genetech, Tecnopharma: Consultancy, Honoraria; Sutro, Takeda: Research Funding; Genentech, AbbVie, Janssen: Consultancy, Research Funding; Novartis: Research Funding; Incyte, celgene: Consultancy; Tecnofarma SAS, AbbVie: Honoraria; Janssen: Honoraria; Incyte, TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Heidelberg Pharma: Research Funding; Amgen: Research Funding. Sborov: Sanofi: Consultancy; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; SkylineDx: Consultancy; GlaxoSmithKline: Consultancy. Reeves: Incyte Corporation: Honoraria; Takeda: Honoraria; Bristol-Myers Squibb: Speakers Bureau; Pharma Essentia: Consultancy, Honoraria. Rodriguez: Karyopharm: Consultancy, Speakers Bureau; Oncopeptides: Consultancy, Honoraria; Amgen: Consultancy, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau. Chari: Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Research Funding; Antengene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Shattuck Labs: Consultancy, Membership on an entity's Board of Directors or advisory committees; Secura Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Consultancy, Membership on an entity's Board of Directors or advisory committees; Millenium/Takeda: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; GlaxoSmithKline: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi Genzyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding. Silbermann: Sanofi Genzyme: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Anderson: Celgene, BMS, Janssen, GSK, Karyopharm, Oncopeptides, Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Shah: Sutro Biopharma: Research Funding; Janssen: Research Funding; Indapta Therapeutics: Consultancy; CareDx: Consultancy; Sanofi: Consultancy; Kite: Consultancy; Poseida: Research Funding; Amgen: Consultancy; BMS/Celgene: Research Funding; Bluebird Bio: Research Funding; CSL Behring: Consultancy; GSK: Consultancy; Precision Biosciences: Research Funding; Teneobio: Research Funding; Oncopeptides: Consultancy; Nektar: Research Funding; Karyopharm: Consultancy. Bumma: Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees. Holstein: Oncopeptides: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech, GSK, Janssen, Secura Bio, Sorrento: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Jakubowiak: BMS: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Gracell: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees. Wildes: Carevive: Consultancy; Seattle Genetics: Consultancy; Sanofi: Consultancy; Janssen: Consultancy. Orlowski: CARsgen Therapeutics, Celgene, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Other: Clinical research funding; Asylia Therapeutics, Inc., BioTheryX, Inc., and Heidelberg Pharma, AG.: Other: Laboratory research funding; Asylia Therapeutics, Inc.: Current holder of individual stocks in a privately-held company, Patents & Royalties; Amgen, Inc., BioTheryX, Inc., Bristol-Myers Squibb, Celgene, Forma Therapeutics, Genzyme, GSK Biologicals, Janssen Biotech, Juno Therapeutics, Karyopharm Therapeutics, Inc., Kite Pharma, Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, I: Membership on an entity's Board of Directors or advisory committees; Amgen, Inc., BioTheryX, Inc., Bristol-Myers Squibb, Celgene, EcoR1 Capital LLC, Genzyme, GSK Biologicals, Janssen Biotech, Karyopharm Therapeutics, Inc., Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, Inc., Sanofi-Aventis, and Takeda P: Consultancy, Honoraria. Shain: Novartis Pharmaceuticals Corporation: Consultancy; Karyopharm Therapeutics Inc.: Honoraria, Research Funding; Janssen oncology: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi Genzyme: Consultancy, Speakers Bureau; GlaxoSmithLine, LLC: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Adaptive Biotechnologies Corporation: Consultancy, Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding. Cowan: Janssen: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Sanofi: Consultancy, Research Funding; Cellectar: Consultancy; Harpoon: Research Funding; GSK: Consultancy; Secura Bio: Consultancy; BMS: Research Funding; Nektar: Research Funding. Dholaria: Takeda: Research Funding; Jazz: Speakers Bureau; MEI: Research Funding; Angiocrine: Research Funding; Poseida: Research Funding; Celgene: Speakers Bureau; Pfizer: Research Funding; Janssen: Research Funding. Pei: Janssen: Current Employment, Current equity holder in publicly-traded company. Cortoos: Janssen: Current Employment, Current equity holder in publicly-traded company. Patel: Janssen: Current Employment. Bartlett: Janssen: Current Employment. Vermeulen: Janssen: Current Employment, Current equity holder in publicly-traded company. Lin: Janssen: Current Employment. Richardson: AstraZeneca: Consultancy; Regeneron: Consultancy; Celgene/BMS: Consultancy, Research Funding; Oncopeptides: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; AbbVie: Consultancy; GlaxoSmithKline: Consultancy; Karyopharm: Consultancy, Research Funding; Protocol Intelligence: Consultancy; Janssen: Consultancy; Sanofi: Consultancy; Secura Bio: Consultancy; Jazz Pharmaceuticals: Consultancy, Research Funding. Voorhees: Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Secura Bio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
The specific regimen combination is not yet approved, but individual components are.